Tiny particles; huge impact: Quantum dots and their discovery – Carl Roth
Quantum dots offer enormous potential for a whole host of applications from engineering to biomedicine, winning their discoverers the prestigious Nobel Prize in Chemistry this year. Read our article to find out how they were discovered and synthesised, and what makes these tiny particles so special.
It is part of human nature for us to intuitively understand things that seem logical, following a kind of primal instinct. For instance, water is fluid and creates waves; stones are hard and do not. Logical. Really? In the world of quantum physics, things work differently. Once we start measuring the size of matter in nanometres (millionths of a millimetre), strange things, known as quantum effects, start to happen, challenging our intuition. Physical quantities can no longer be determined precisely, and particles can simultaneously be waves. Yet the physical structure of matter, in terms of its atomic composition, remains the same, which means nanoscopic matter must also be part of our “normal” world, even if we cannot understand it.
Fortunately, the desire to understand things, especially when they seem illogical, is the defining characteristic of the scientist! This year, the Nobel Prize in Chemistry was awarded to Moungi Bawendi, Louis Brus and Alexei Ekimov for their courageous scientific work. Through their endeavours, they have, to a certain extent, brought the quantum world into our everyday lives.
The tiniest miracles behind myriad applications
Our ancestors have been making use of quantum dots for a long time, without even knowing they exist – the brightly coloured glass in church windows, known and used for centuries, gets its colour from the quantum effects of the tiny particles that are mixed into the glass. Nowadays, quantum dots are true all-rounders in terms of their applications. Modern Ultra HD televisions, for example, are unimaginable without quantum dots, as these optimise the backlighting by expanding the colour space. The further development of future display technologies is sure to also benefit greatly from research into quantum dots. Quantum dots can act as efficient emitters of light, and are used, for example, in the production of modern laser diodes. They can also be used in photovoltaics to convert sunlight into electrical energy more efficiently, thereby enabling solar cells to be thinner. Biomedicine is another application where quantum dots are used, here to mark (diseased) tissue, to make tumours visible, and enable surgeons to clearly distinguish cancerous tissue from healthy tissue, for example. Quantum dots can also serve as quantum bits (qubits), which are used in quantum information processing. This means they can be used as the basis for the future development of quantum computers and secure quantum communication systems. Last but not least, quantum dots can be used as highly sensitive sensors to measure various physical or chemical quantities. They can be used to detect molecules, gas concentrations or temperature changes.
There seems to be no end to either the possible applications or the economic potential of quantum dots, deemed immense by those in the know. “Quantum dots are thus bringing the greatest benefit to humankind”, stated the Nobel Prize Committee in its decision. So, what makes these particles so special?
Quantum effects arise when particles shrink
First of all, quantum dots, whose discovery and synthesis by Moungi G. Bawendi from the Massachusetts Institute of Technology (MIT), Louis E. Brus from Columbia University in New York, and Alexei I. Ekimov, formerly of Nanocrystals Technology Inc. in New York, won them the 2023 Nobel Prize in Chemistry, are simply crystals made of semiconductors. A semiconductor is a material that can be both insulating (at low temperatures) and conductive (at high temperatures). The special thing about quantum dots is their size: these are nanoscopic crystals measuring just 2 to 12 nanometres. Even a coronavirus is ten times larger, measuring 120 to 160 nanometres. Ten nanometres puts us in the same range as large biological molecules such as haemoglobin – the radius of many atoms is approximately one angstrom (Å), 0.1 nanometres, so 100 times smaller.
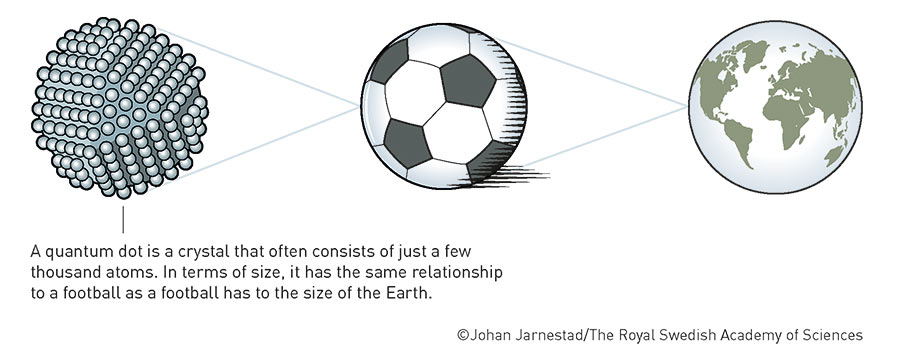
The small size of these crystals means that the mobility of charge carriers in quantum dots is restricted in all three spatial directions. As a result, the energy of these charge carriers can no longer assume arbitrary continuous values, but only discrete values, i.e. numbers with a finite number of digits after the decimal point. This results in the quantum dots exhibiting the same quantum behaviour as individual atoms, thereby forming a new class of materials that is neither molecular nor bulk. Although they have the same structure and atomic composition as conventional materials, their properties can be controlled by a single parameter: the size of the particles. Thanks to this (theoretically) simple mechanism, not only can the optical and electronic properties of the nanoscopic crystals be tailored, so can, for example, their redox potential, their melting temperature, or solid-state phase transitions.
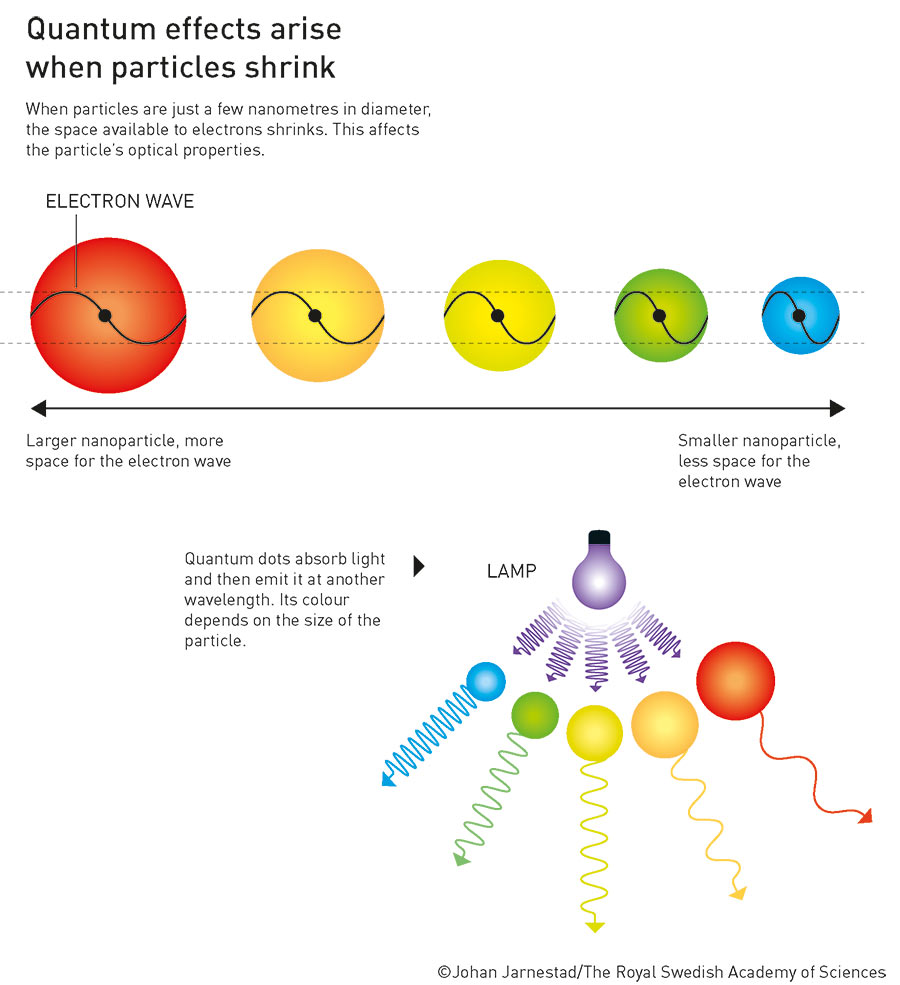
What exactly did the Nobel Prize winners discover?
It has long been postulated in theoretical physics that size-dependent quantum effects occur in nanoparticles. However, for a long time, it was not possible to specifically locate these nanoparticles. Any kind of practical application with predictable effects was therefore equally impossible.
That all changed in the early 1980s, when Alexei Ekimov and Louis Brus – independently of one another due to the Cold War – observed size-dependent quantum effects in their experiments. Ekimov, who carried out his research in the Soviet Union, managed to show through examinations of coloured glass that its colour was determined by the size of the coloured particles contained in the glass. At the same time, but in the USA, Louis Brus was researching chemical reactions that would make sunlight usable. As is often the case, chance was a factor here, with Brus discovering that the cadmium sulphide solutions used for this changed their optical properties over longer periods of time. More detailed investigations confirmed what Ekimov had also found: the size of the particles in the solution determined their colour. The change which occurred when these were left to stand over time was later explained by the phenomenon known as Ostwald ripening, already described by Wilhelm Ostwald in 1900.
Brus had produced quantum dots without intending to. What neither Brus nor Ekimov was able to do, however, was to specifically produce very high quality quantum dots using a simple and inexpensive synthesis process. It was one of Brus’ former doctoral students, Moungi Bawendi, who finally succeeded in opening the door to the world of everyday applications. In 1993, he published a refined process in the Journal of the American Chemical Society which allowed him to specifically produce nanocrystalline semiconductors in sizes between just over one and 11.5 nanometres. To do this, he used organometallic compounds that he injected into very hot solvent, causing it to cool suddenly. The key was a slow and controlled reheating of the solution, which allows the previously formed crystal nuclei to begin to grow in a controlled and uniform manner.
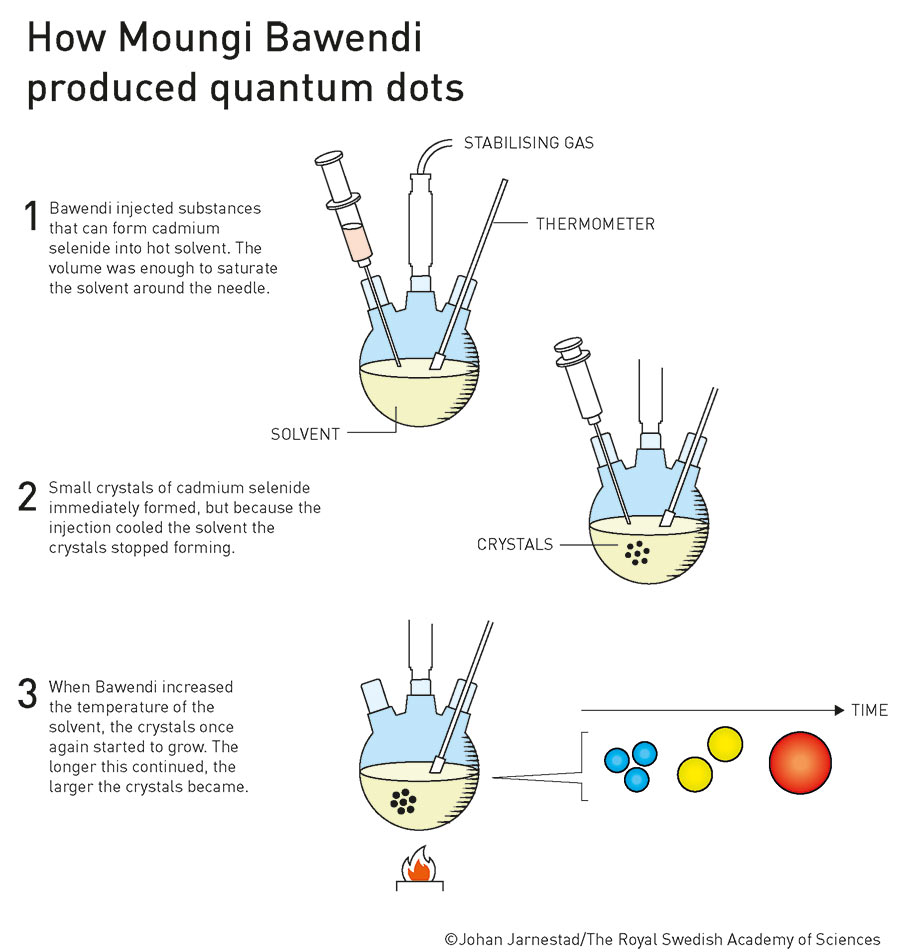
The desired size of the crystals could be easily and specifically controlled via the temperature to create almost perfect quantum dots. Together with the preparatory work of his two colleagues, this was the decisive breakthrough that triumphantly opened up a world of possibilities for quantum dots. To this day, every year sees new, sometimes groundbreaking applications of these nanoscopic structures being introduced, based on the work of the three pioneers. A Nobel Prize-worthy discovery, concluded the Royal Swedish Academy of Sciences selection committee.
Sources:
https://www.nobelprize.org/uploads/2023/10/advanced-chemistryprize2023-3.pdf
https://www.nobelprize.org/uploads/2023/10/popular-chemistryprize2023-3.pdf
https://www.spektrum.de/news/chemie-nobelpreis-2023-die-quantenpunkte-aus-der-zwischenwelt/2186634
https://www.deutschlandfunk.de/nobelpreis-chemie-2023-100.html


