A crucial step ahead of the curve: What made mRNA vaccines possible – Carl Roth
mRNA technology offers enormous potential, even beyond vaccine development, helping its pioneers win the Nobel Prize in Physiology or Medicine this year. Read this article to find out how this technology enabled the extremely rapid development of the COVID-19 vaccines, and what clinical applications can also be expected from it in the future.
The development of vaccines is always a race against time. This was particularly true when SARS-CoV-2 emerged at the end of 2019 and quickly spread to all parts of the world. Few thought that the rapid and, in some countries, devastating development of the coronavirus pandemic could be quickly countered with highly effective vaccines. However, this is actually exactly what was achieved: a number of vaccines were brought to market in record time, thereby saving countless lives.
Two of the most effective vaccines, and the fastest to be approved, are based on so-called mRNA technology. The idea behind this has been around for over 30 years, but crucial hurdles initially prevented its clinical application. It was not until Katalin Karikó and Drew Weissman started their work that a breakthrough was made – and the two researchers have now received the Nobel Prize for Physiology or Medicine in 2023. It is largely thanks to the results of their research that the so-called mRNA vaccine platform was ready for clinical use when it was so urgently needed, with this also paving the way for future mRNA applications. But what exactly did Karikó and Weissman discover? To help explain, let’s take a brief look at the theory and history of vaccine development.
The principle of vaccination and vaccines before the pandemic
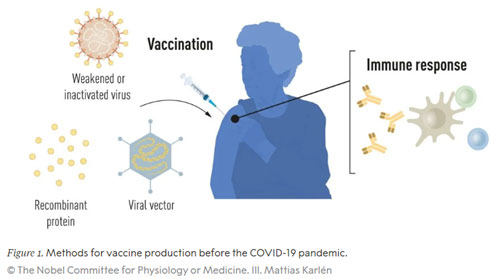
A vaccination induces an immune response against a specific pathogen. First of all, the immune system responds quickly and specifically to the corresponding antigen on the pathogen. This is then followed by the formation of so-called memory cells. Should this pathogen be encountered again, these cells are the key to ensuring that the immune system is already prepared. Immunity has been built up against the pathogen, meaning the appropriate antibodies can be formed immediately, with these in turn fighting the infection in a specific immune response.
Due to experiences from the first smallpox vaccination and the discovery of bacteria and viruses as pathogens, the late 19th and especially the 20th century were the golden age of targeted vaccine development. In 1881, Louis Pasteur found a vaccine against anthrax, and in 1913, Emil von Behring developed a vaccine against diphtheria. These were followed by vaccines against tetanus and pertussis (whooping cough) (1926), yellow fever and influenza (flu) (1936), and poliomyelitis (polio) (1955 and 1961). In the 1960s, vaccines against measles, mumps and rubella then became available in Germany. In addition to the discovery of antibiotics, this vaccine development based on killed pathogens is one of the most important milestones in human medical history, and has saved countless people from death or disfigurement. In 1951, Max Theiler received the Nobel Prize in Physiology and Medicine for the development of the yellow fever vaccine.
https://www.nobelprize.org/prizes/medicine/2023/press-release/
Most of the antiviral vaccines approved today are still produced using traditional methods based on weakened or inactivated whole viruses. These take a lot of time and resources to produce: as the virus cannot replicate on its own, but must rely on the DNA/RNA synthesis machinery of host cells, vaccines are traditionally produced in chicken eggs or cell cultures. A time-consuming process! This method does not allow for a quick response to outbreaks and pandemics.
With advances in molecular biology and the development of technologies for producing recombinant proteins, new opportunities emerged for more targeted vaccine approaches that are no longer based on whole virus particles (virions), but only on the antigens to which the immune system responds. The first vaccine produced in this way was the hepatitis B (HBV) vaccine, approved in 1986, which was followed in 2006 by the approval of the first vaccine against (cancer-causing) human papillomavirus (HPV). The HBV and HPV vaccines contain individual protein components from the respective virus, and are referred to as subunit vaccines. However, these vaccines also require the use of cell cultures for their production, subject to the corresponding restrictions inherent in this resource-intensive process. For this reason, researchers have long been trying to develop vaccine technologies which do not rely on cell cultures, with mRNA technology proving a promising option.
mRNA vaccines: A concept with far-reaching potential
The genetic information encoded in the DNA in our cells is transferred to messenger RNA (mRNA), which serves as a template for protein biosynthesis. In the 1980s, efficient methods for producing mRNA without cell culture, known as in vitro transcription, were introduced. This crucial step sped up the development of molecular biology applications in a range of areas. The concept of using these new molecular biology techniques to produce mRNA-based vaccines, or to treat diseases through the administration of mRNA by replacing defective genes with functional ones, or overexpressing a therapeutic protein, aroused great interest. But there were some crucial obstacles to overcome. In vitro-transcribed mRNA proved unstable, difficult to administer, and led to inflammatory responses in the body, meaning that sophisticated carrier lipid systems had to be developed to encapsulate the mRNA. This dampened any initial enthusiasm towards developing mRNA technology for clinical purposes. However, it did not discourage Hungarian biochemist Katalin Karikó and her colleague, immunologist Drew Weissman, who continued to research their idea of developing methods to use mRNA for clinical applications. Both were conducting research at the University of Pennsylvania in the early 1990s, and a fruitful collaboration began.
How Karikó and Weissman achieved their breakthrough
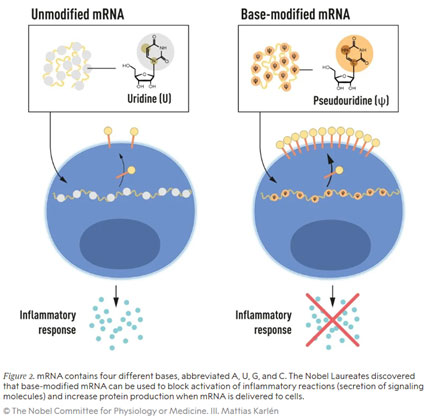
Karikó and Weissman were able to show that dendritic cells – highly specialised antigen-presenting cells in the immune system that initiate and regulate antigen-specific immune responses – recognise the administered in vitro transcribed mRNA as a foreign substance, which leads to cell activation and the release of inflammatory signals. They wondered why only mRNA transcribed in vitro triggered this response, but mRNA produced in mammalian cells did not. It was already known that the nucleoside bases of RNA from mammalian cells are often chemically modified, whereas those of mRNA from in vitro transcriptions are not. Could it be the absence of these post-transcriptional base modifications causing the unwanted inflammatory response to mRNA produced in vitro? In order to investigate this, Karikó and Weissman produced different mRNA variants in vitro, each with a defined chemical change in the bases, and applied them to dendritic cells. The results were astonishing. The inflammatory response disappeared almost entirely when either 5-methylcytidine (m5C), N6-methyladenosine (m6A), pseudouridine (ψ), 5-methyluridine (m5U), or 2-thiouridine (s2U) was incorporated in the in vitro transcribed mRNA.
https://www.nobelprize.org/prizes/medicine/2023/press-release/
Not only was this a paradigm shift in understanding how cells recognise and respond to different forms of mRNA, Karikó and Weissman also immediately saw that their discovery would have profound implications for the use of mRNA in therapy. Their groundbreaking results were published in 2005, fifteen years before the COVID-19 pandemic. In further studies, published in 2008 and 2010, the two researchers showed that in vitro transcribed, nucleoside-modified mRNA also significantly increased protein production compared to unmodified mRNA – another important component for use in medical therapies. This removed the two crucial obstacles that had previously prevented mRNA from being tested for clinical applications.
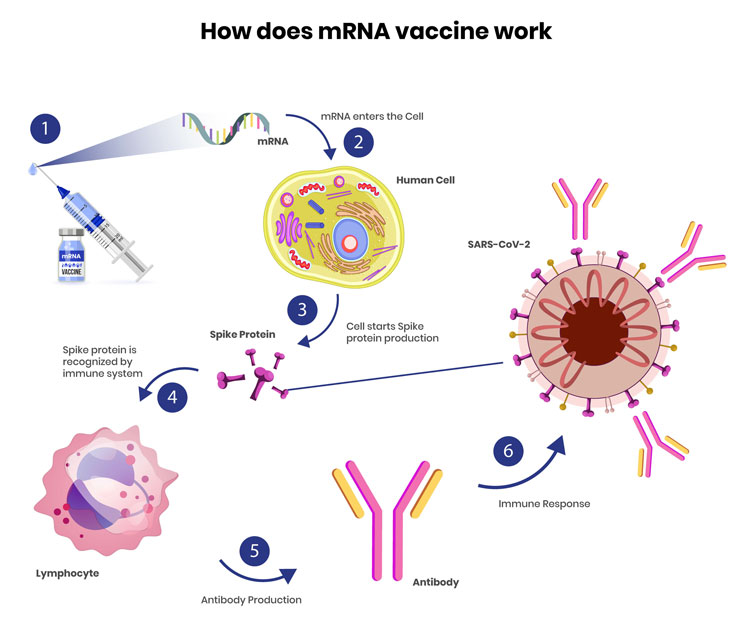
mRNA technology: From saving lives in the pandemic to a highly promising future
To date, researchers have uncovered more than a hundred different post-transcriptional modifications in RNA. Later work showed that the use of N1-methylpseudouridine (m1ψ), alone or in combination with the methylcytidine (m5C) described above, further improved the mRNA platform, both in terms of reducing innate immune receptor recognition and increasing protein expression.
Interest in mRNA technology began to grow, and by 2010 there were three major players active within this market: CureVac, BioNTech and Moderna. They worked on further developing this method and its therapeutic applications, developing, among other things, vaccines against the Zika virus and MERS-CoV, a virus closely related to SARS-CoV-2. Following the outbreak of the COVID-19 pandemic, these companies were able to respond quickly and developed two nucleoside-modified mRNA vaccines which encoded the SARS-CoV-2 surface protein in record time using m1ψ. In clinical trials, these vaccines demonstrated protective immunity of around 95%, and both vaccines were approved in December 2020.
To date, around 15 billion doses of COVID-19 vaccines have been administered worldwide, and the vaccine is continually being adapted to the latest Sars-CoV variants. It would not have been possible to develop this during one of the greatest health crises of our time without the work of this year’s Nobel Prize winners in Physiology or Medicine. The impressive speed and flexibility with which new mRNA vaccines can be developed has also paved the way for the new platform to be used in future for vaccines against other infectious diseases. Many experts assume that mRNA technology will be the dominant vaccination platform in around 20 years. Furthermore, the technology could also be used in the future to build therapeutic proteins in patients and to treat some types of cancer. In a recent press release, BioNTech announced that it had demonstrated the effect of a vaccination against cancer on humans for the first time. The idea behind it is that it teaches the immune system to recognise cancer cells as foreign, even though they are the body’s own. Tumour cells also form antigens that could trigger an immune response, but these cells are very good at hiding from the body’s own immune system. The basis of the vaccination presented here is a combination of cells known as CAR-T cells (“chimeric antigen receptor” T cells) and an mRNA vaccine. These are artificially modified immune cells that are generated from the patient’s own cells. They produce a specific protein (chimeric antigen receptor) and express it on their surface. This “antenna” ensures that the CAR-T cells recognise the patient’s cancer cells, bind to them precisely using the lock-and-key principle, and destroy them. This strategy shows great promise for some cancers, but is expensive and time-consuming. The BioNTech work shows that the mRNA vaccine can enable immune cells to generate the chimeric antigen receptor themselves, quickly and elegantly replacing the time-consuming and expensive modification of these cells in the laboratory. The combination of CAR-T cells and mRNA lays the foundation for the corresponding cancer cells in the body to be eliminated as soon as they have developed.
The development initiated by Nobel Prize winners Karikó and Weissman is far from over. In our globalised world, it is not unlikely that further pandemics will break out, and cancer has always been one of the great scourges of humanity. Karikó and Weissman have given us a valuable tool to ensure we are not left defenceless.
Sources:
https://www.nobelprize.org/prizes/medicine/2023/summary/
https://www.nobelprize.org/prizes/medicine/2023/press-release/
https://www.nobelprize.org/uploads/2023/10/press-medicineprize2023-3.pdf
https://www.nobelprize.org/prizes/medicine/2023/advanced-information/
https://www.wienerzeitung.at/a/eine-impfung-gegen-krebs?utm_source=pocket-newtab-de-de
https://www.impfen.de/impfwissen/alle/die-geschichte-der-impfung/