Gene therapy
Even in the age of modern medicine, many serious illnesses are still untreatable. Many of these conditions are genetic. For some years now, gene therapy has been the talk of the town as a new era in medicine. But is it really capable of permanently curing diseases by simply repairing our genetic make-up? Where are the opportunities, the challenges and the risks? We shed light on the state of the art in Germany and Europe.
The human genome has around 3,100,000,000 (3.1 x 1010) base pairs. Just three more base pairs can make the difference between life and death. CAG, short for the three DNA bases cytosine, adenine and guanine, is the fatal sequence in the hereditary, always fatal nervous disease Huntington’s disease, the first genetic disorder for which researchers found the responsible Huntington gene on human chromosome 4 around 30 years ago. In the genetic code of the healthy gene, the sequence CAG is found in 10- to 26-fold repetition. The base triplet codes for the amino acid glutamine, the resulting protein ‘huntingtin’, HTT for short, therefore also contains a polyglutamine area in healthy individuals. However, the pathological Huntington gene grows during cell division and more and more CAG sequences are added – resulting in an altered HTT. From 40 repetitions, it is considered certain that the affected person will fall ill: the more CAG units in direct succession, the earlier the onset of the disease. However, if the chain of CAGs is interrupted by just a single CAA sequence, for example, those affected are spared for longer.
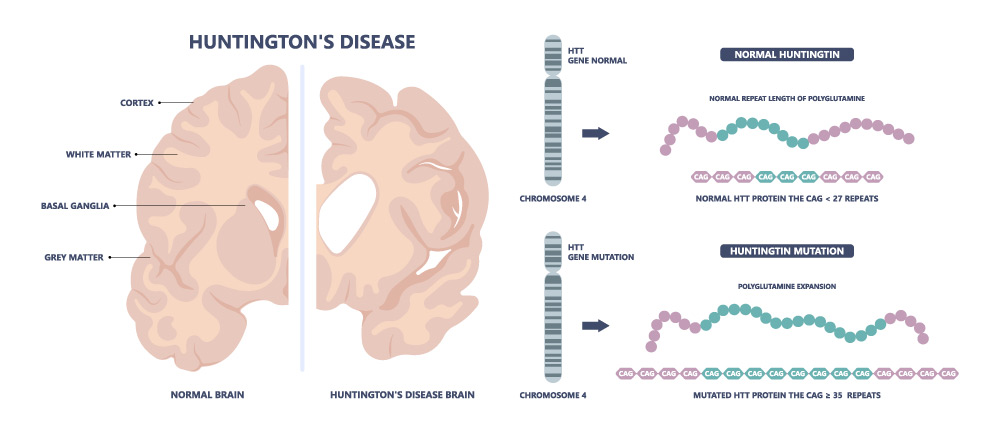
There is hardly any other hereditary disease about which so much is known as Huntington’s disease, which is considered a “rare disease”. The fatal condition now serves as a model disease that researchers are using to learn about other hereditary diseases – but it cannot yet be cured due to the lack of a causal therapy. However, in the age of high-tech medicine, molecular genetics and gene therapy, should it not be possible to “repair” the causal defect in the genetic material? What does gene therapy actually mean and have we even reached the age of gene therapy? For the treatment of which diseases is gene therapy already being used in Germany and what are the main challenges? And can gene therapy really cure hereditary diseases causally instead of just treating symptoms?
According to the definition by the Paul Ehrlich Institute, a gene therapeutic is “a biological medicinal product whose active substance contains or consists of a nucleic acid (carrier of genetic information). It is used to regulate, repair, replace, add or remove a nucleic acid sequence. The therapeutic, prophylactic or diagnostic effect is directly related to the recombinant nucleic acid sequence that it contains or to the product that is formed on the basis of this genetic information.”
According to an elaboration by the German Bundestag in 2024, gene therapeutics are “advanced therapy medicinal products (ATMPs) in accordance with the Medicinal Products Directive 2001/83/EC1 and the ATMP Regulation (EC) 1394/20072. ATMPs are regulated in the EU in the same way as medicinal products and also in accordance with the aforementioned ATMP Regulation. […] For marketing authorisation, ATMPs must undergo the EU’s centralised authorisation procedure at the European Medicines Agency (EMA) like any other medicinal product.”
Gene therapy – euphoria followed by disillusionment
The beginning of this completely new era in medical history was marked on 14th September 1990, when US doctors French Anderson and Michael Blaese treated Ashanti De Silva, then aged four, with retroviral gene therapy for the first time as part of a clinical trial. The girl, who therefore became the world’s first patient to be treated with gene therapy, suffers from the genetic immunodeficiency ADA-SCID: she lacks an enzyme that protects the T lymphocytes, which are so important for immune defence, from attack by another protein. As a result, the T lymphocytes in their bone marrow do not mature or only mature in insufficient numbers, making all pathogens a potentially fatal threat to them. Most patients with this disease do not survive childhood. French Anderson and Michael Blaese removed some of the girl’s remaining white blood cells and added a retrovirus that had previously been genetically modified in the laboratory and equipped with a functioning copy of the defective gene. This acts as a “gene taxi” and introduces the correct gene into the girl’s immune cells. The cells modified in this way were then administered to her again by infusion. Today, there are doubts as to whether a second drug administered to Ashanti contributed to her success – but the girl is now a young woman and alive.
Ashanti’s case triggered euphoria among researchers and doctors. Many saw the dawn of a new era in which the most serious hereditary diseases or other conditions with genetic involvement, such as cancer, immune and metabolic diseases, would be curable. But the euphoria was followed by disillusionment. Of all things, the viral vectors used as “gene taxis” led to severe side effects and complications in some patients. Some experienced severe, excessive and even fatal immune reactions, while others developed leukaemia as a result of the treatment. The “gene taxis” were apparently too unsafe and unspecific.
Safe “taxis” for gene therapeutics at work
Viruses are initially predestined to be “gene taxis”. This is because what they are really good at is introducing their genetic material into other host cells and replicating it there with the help of the host cell’s own machinery. This is the only way they can reproduce.
However, neither of the two systems currently in use works perfectly: the frequently used adenoviruses introduce the genetic material into the cell highly efficiently, but only temporarily, which is why gene therapy treatment based on such vectors usually has to be repeated regularly throughout the patient’s life. In addition, although these vectors reduce the risk of “incorrect insertions” into the patient’s genome, they often trigger strong immune reactions as (modified) pathogens of, for example, influenza infections. Retroviruses, on the other hand, permanently insert the therapeutic gene into the genome – ideally, a single injection of the gene therapeutic can be sufficient to correct the genetic defect and cure the disease. However, the genetic material is sometimes inserted several times and unspecifically elsewhere in the genome, which can ultimately also cause cancer.
New ways and vectors for the safe, efficient and targeted introduction of genetic information into patients’ cells were therefore urgently needed and are still the subject of intensive research today. Advances in the field of genetic engineering in particular, most recently the CRIPRS/Cas9 technology, have made it possible to design vectors in an increasingly targeted manner. In this way, the safety and specificity of some viral vectors have been continuously increased, particularly in the last two decades, which has led to a kind of renaissance.
One approach has been to reduce the size of these vectors to just the functions required for efficient gene transfer, while at the same time increasing the specificity of the insertion. The first gene therapeutic, which received official marketing authorisation for Europe on 2nd November 2012, is based on an enucleated mini-virus, the so-called adeno-associated virus (AAV). These extremely small viruses leave their genetic material (with the gene therapeutic) as an extrachromosomal piece of DNA in the nuclei of the target cells, where it is read by the cell’s own machinery. In this case, the preparation “Glybera”, developed by the Amsterdam-based company UniQure, should help patients suffering from the hereditary metabolic disease lipoprotein lipase deficiency (LPLD). The disease causes certain blood lipids in those affected to rise abnormally. However, the drug was withdrawn from the market for economic reasons.
Gene therapy – state of the art in Germany
There are basically two different approaches to gene therapy.
The modification of the genetic material of body cells (somatic cells) is limited to individual patients (somatic gene therapy). The treated gene sequence is not passed on to their offspring. If, on the other hand, the genetic material of germ cells (sperm or eggs or their precursors) is interfered with, not only the genetic material of the individual patients is changed, but also that of their offspring (germline therapy). As germline therapy harbours a higher risk of unforeseeable consequences, it requires more comprehensive technical and ethical assessments. In Germany, it is prohibited under Section 5 of the Embryo Protection Act (ESchG). All gene therapies that have been developed and tested in clinical trials to date are somatic gene therapies.
With regard to the introduction of the desired therapeutic gene or the tool for repairing genes into the right cells, a distinction is made between in vivo approaches, in which the therapeutic agent is administered directly and the target cells are targeted directly in the organism, and so-called ex vivo approaches. Here, cells, for example stem cells from the bone marrow, are removed from the target person, genetically modified in the laboratory and then re-administered. A distinction is also often made between in situ approaches, in which the therapeutic agent is introduced directly into the affected organ or a specific site.
The ex vivo strategy is also known as cell-based gene therapy and is used in cancer therapy in particular. In the case of CAR T-cell therapy, T lymphocytes are removed from patients and genetically modified so that they can recognise and attack cancer cells via specific antigen receptors on their surface. What is remarkable about this so-called N=1 therapy is that the therapeutic agent is tailored to a single target person (number ‘N’ = 1) and can therefore have the optimum effect. This so-called cancer immunotherapy is considered extremely promising for the treatment of certain types of cancer. In Germany, 6 of the 16 approved gene therapies are currently CAR T-cell therapies (see table). Intensive research is being carried out in this field, which is why further authorisations can be expected in the future. In addition to malignant diseases such as leukaemias and lymphomas, congenital diseases of the blood coagulation system, such as haemophilia A and B, are another main area of application for gene therapeutics currently approved in Germany.
The vast majority of all gene therapeutics approved in Germany are aimed at so-called “rare diseases”. As around 80 per cent of these are genetically determined or co-determined, gene therapy is considered to have great potential for improving the care of those affected. In particular, monogenetic diseases, i.e. those for which only a single gene is responsible, are considered to be addressable by gene therapy and are the subject of intensive research in some cases.
A disease is considered “rare” in this country if no more than one in 2000 EU citizens suffers from it. Among other things, companies can apply for orphan drug status for active substances developed to treat these diseases. Although this does not lead to an accelerated procedure for drug approval, authorisation holders receive a number of benefits, such as ten years of market exclusivity within the EU.
Cause for hope even with Huntington’s disease?
And Huntington’s? Can those affected also hope for a gene therapy treatment option in the future? This rare disease also has a monogenetic cause and is also well researched. In fact, many companies and researchers are currently working on possible gene therapies to correct or “switch off” the affected Huntington’s gene.
The most promising drug candidate at present is probably AMT-130 from uniQure – the company that received the first market approval for a gene therapy in Europe in 2012. Last summer, the company announced in a mid-term review that its gene therapy had clearly delayed the decline of the test subjects in two clinical phase I/II trials after two years of observation: at a low dose by 30 per cent, but at a higher dose by a full 80 per cent, which is almost equivalent to a standstill of the disease. This news triggered cautious optimism in the “Huntington’s scene” – when an effect is so clearly dependent on the dose of an active ingredient, this is considered a good sign in pharmaceutical research and development that the measured effect is real.
According to the company, the gene therapy product candidate AMT-130 consists of an AAV5 vector (adeno-associated virus type 5), which carries an artificial microRNA that prevents the reading of the Huntington’s gene in the patient’s cells by means of a non-selective knockdown. The therapeutic aim is therefore to inhibit the production of the mutated protein mHTT, which is toxic to the nerve cells of those affected. The US Food and Drug Administration (FDA) apparently also considers the drug to be so promising that it was the first therapeutic candidate to be granted RMAT status for Huntington’s disease (Regenerative Medicine Advanced Therapy). RMAT status enables increased collaboration with the FDA in order to accelerate development and possibly achieve faster approval. The drug has also already been granted orphan drug status.
Huntington’s disease therapy is made particularly difficult by the fact that the disease takes place in certain cells of the brain of those affected and the drug has to get exactly there. However, getting a drug into the brain and therefore overcoming the so-called blood-brain barrier is anything but trivial – gene correction in the nerve cells of the brain is even more difficult. At present, AMT-130 must therefore be injected directly into the brain of those undergoing treatment, which they accept in view of the hopelessness of this disease.
However, the list of serious diseases that are still almost impossible to treat is long. Around 8000 diseases worldwide are among the rare diseases that are under-represented in medicine for various reasons. In many cases, they cause enormous suffering; neither the disease nor any kind of therapy is a “walk in the park”. If there is a legitimate chance of relief or a cure, gene therapy can be a very individual hope and decision.
Gene therapy medicinal products authorised in Germany (as of 14th March 2025; source: Paul Ehrlich Institute, Gene therapy medicinal products – Paul Ehrlich Institute)
| Designation | Authorisation /approval holder | Indication | Authorisation/approval data | Explanations |
|---|---|---|---|---|
| Abecma | Bristol-Myers Squibb Pharma EEIG | Multiples Myelom | 18.08.2021 | Rare disease, CAR T-cell therapy |
| Beqvez (previously Durveqtix) | Pfizer Europe MA EEIG, Belgien | Haemophilia B | 24.07.2024 | Rare disease |
| Breyanzi | Pharmaceuticals (Ireland) Limited | Diffuse large B-cell lymphoma (DLBCL); High-grade B-cell lymphoma (HGBCL); Primary mediastinal large B-cell lymphoma (PMBCL); Follicular lymphoma grade 3B (FL3B) | 04.04.2022 | CAR T-cell therapy |
| Carvykti | Janssen-Cilag International N.V. | Relapsed or refractory multiple myeloma | 25.05.2022 | Rare diseases First CRISPR therapy |
| Casgevy | Vertex Pharmaceuticals (Ireland) Limited | Beta-thalassaemia and sickle cell anaemia | 09.02.2024 | Rare diseases First CRISPR therapy |
| Hemgenix | CSL Behring GmbH | Haemophilia B | 20.02.2023 | Rare disease |
| Imlygic | Amgen Europe B.V. | Melanoma | 16.12.2015 | Oncolytic virus |
| Kymriah | Novartis Europharm Ltd., IRL | Acute lymphoblastic B-cell leukaemia and diffuse large B-cell lymphoma | 23.08.2018 | Rare diseases, CAR T-cell therapy |
| Libmeldy | Orchard Therapeutics (Netherlands) B.V., NL | Metachromatic leukodystrophy | 17.12.2020 | Rare disease |
| Luxturna | Novartis Europharm Limited | Retinal dystrophy | 22.11.2018 | Rare disease |
| Roctavian | BioMarin International Limited, Irland | Haemophilia A | 24.08.2022 | Rare disease |
| Strimvelis | Fondazione Telethon ETS | Adenosin-Desaminase-Mangel (ADA-SCID) | 26.05.2016 | Rare disease |
| Tecartus | Fondazione Telethon ETS | Relapsed or refractory mantle cell lymphoma | 14.12.2020 | Rare disease, CAR T-cell therapy |
| Upstaza | PTC Therapeutics International Limited, Irland | Aromatic l-amino acid decarboxylase (AADC) deficiency | 18.07.2022 | Rare disease |
| Yescarta | Kite Pharma EU B.V., NL | High-grade B-cell lymphoma (HGBL); Diffuse large B-cell lymphoma (DLBCL); Primary mediastinal large B-cell lymphoma (PMBCL); Follicular lymphoma (FL) | 23.08.2018 | Rare diseases. CAR T-cell therapy |
| Zolgensma | Novartis Europharm Limited, Dublin | Spinal muscular atrophy (SMA) | 18.05.2020 | Rare disease |
Sources:
Gentherapeutika – Paul-Ehrlich-Institut
Gentherapie | Seltene Erkrankungen
Meldungen – Erstes Gentherapeutikum gegen Hämophilie A erhält Zulassung – Paul-Ehrlich-Institut
Chorea Huntington – Symptome, Diagnostik, Therapie | Gelbe Liste
Korrekturen an Chromosom 4: Gentherapien sollen Huntington-Krankheit verhindern
Programme & Pipeline | uniQure
Orphan Drug-Status im AMNOG-Verfahren erklärt | vfa
Die Gen-Reparateure – scinexx.de
Vergleich der regulatorischen Anforderungen an Gentherapeutika und genbasierte Impfstoffe

